Pauling electronegativity
Pauling noticed that B(A-B) is almost always larger than the mean of the homonuclear A-A and B-B bond energies and attributed this to the opportunity of ionic-covalent resonance involving valence structures like A+B- when B is the more electronegative atom. He associated the bond strengths to the electronegativities xA and xB of the two elements as per the formula
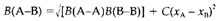
In which the constant C takes the value 96.5 if B values are in kj mol-1. According to this this formula depends only on the variation of electronegativities, it is essential to choose one value to start the scale; Pauling chose 4.0 for the electronegativity of fluorine.
Pauling's formula should be considered as purely empirical and without any rigorous theoretical foundation. Though, the electronegativity scale is extensively used and depicts similar trends as ones based more directly on atomic quantities. Pauling's formula provides a helpful rationalization of some bond-strength trends and can be employed as a semi quantitative guide for estimating unknown bond enthalpies. It should not be employed for solids with a high degree of ionic character.