Bond enthalpies
The simplest measure of the strength of a bond is the energy needed to break it. Estimates of such type of bond energies are generally obtained from thermochemical cycles using Hess' Law and are called bond enthalpies. In a molecule, a bond dissociation enthalpy is the enthalpy change involved in breaking the bond to one atom and in a diatomic is by definition equal to the bond enthalpy. So the enthalpy of dissociation of O2 gives directly the (double) bond enthalpy B(O=O).
While a molecule consists of various equal bonds, the enthalpy needed to dissociate them successively is not similar.
In spite of dealing with individual bond dissociation energies, it is general to define the mean bond enthalpy. So B (O-H) is defined as half the enthalpy change in the process
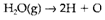
When various types of bonds are involved it is essential to make assumptions about the energies of some of them. For instance, it is general to assume that the value of B(O-H) obtained from H2O can also be applied to H2O2. Then for process
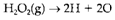
We Have
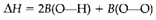
By which the (single) bond enthalpy B(O-O) can be obtained. This quantity is not similar as ΔH for the dissociation of H2O2 into 2OH, as it is disputed that the bonding within the hydroxyl radical OH has changed from the 'normal' situation where oxygen creates two bonds. The supposition of transferability involved in this method of determining bond enthalpies is; though, free to question.