Toxic and medicinal elements
Also listed in Table 1 are some remarkably toxic elements. Toxicity is a comparative term, and several necessary elements are toxic either in excess (example Fe) or if available in the wrong chemical form (example elemental P or Cl). Several of the extremely toxic elements listed in Table 1 are heavy metals, i.e., post-transition elements of periods 5 and 6. These elements contain strong complexing ability and an particularly strong affinity for sulfur. They might displace necessary elements like Ca and Fe, and might also disrupt protein structure through breaking S-S bridges. Once attached to appropriate ligands they are difficult to displace. Chelation therapy is a treatment for heavy metal poisoning by using chelating ligands which bind very much strongly and can eliminate the elements in complexed form.
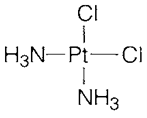
Metallic elements employed in medicine involve lithium for treating manic depressive illness, platinum complexes like cis-platin (2) that act like antitumor agents through combining along with DNA and reducing cell division and gold compounds used to treat arthritis.
Radioactive isotopes are ususally harmful to life due to the damaging effects of ionizing radiation. Elements which are retained through the body and/or concentrated in particular organs (example Pu in the liver and in bones, I in the thyroid gland) are particularly dangerous. Alternatively, several radioactive isotopes are employed in medicine for diagnostic (tracer) and sometimes therapeutic (cancer treatment) purposes. One of the most helpful is technetium (Tc), an artificially made element without stable isotopes. The synthesis of Tc complexes intended to 'target' specific organs in the body is an active research area.