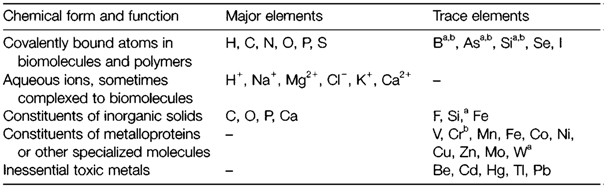
Major elements
Most important elements of life (apart from P) are readily presented in sea water, that may resemble the environment where life began. They fulfill three major functions. Nonmetallic elements (apart from Cl) are components of covalently bound ions and molecules. H, C, N, O and often S are constituents of proteins, and nucleic acids (DNA and RNA) consist of P as well. The chemical forms of these elements different. S is generally exist in reduced (R-S-H) form (R denoting organic
anecassary for some species, not necessarily humans.
bChemical form unknown.
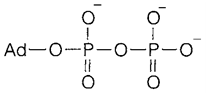
groups), but the facile oxidation to R-S-S-R provides disulfide bridges, that play a structural role in proteins. P, alternatively, is all the time fully oxidized as phosphate. DNA is necessarily made of phosphate diesters (RO)2PO2-complexed along with Mg2+. Adenosine di- and triphosphate, ADP (1: the organic part represented Ad) and ATP, correspondingly, are employed in energy storage in all types of cell. The forward reaction:

has ΔG approximately +35 kJ mol-1 under physiological circumstances of concentration and pH. Metabolic energy input (example from oxidation of glucose) is employed to drive it. The opposite reaction then gives energy for essential functions like muscle contraction or the action of the ion pumps that are mentioned below. An active human might turn over an amount of ATP equal to his or her body weight every day.
Bicarbonate and phosphate ions are also available in aqueous solution and act like a buffers to preserve pH. Metal cations are available in aqueous solution, and are frequently complexed to anionic biomolecules. For instance, Mg2 + is necassary for the function of DNA and for the synthesis and utilization of ATP. Unique 'ion pumps' keep much lower concentrations of Na+ and Ca2+ under cells than in extracellular fluids and local changes in the concentrations of these two cations are employed for signaling. The passage of nerve signals is related with an influx of Na+. Ca2+ forms complexes along with carboxylate groups and acts to change the conformation of several macromolecules; particularly this ion plays a role in muscle contraction.
Other role for some elements is in inorganic solids. Internal skeletons (teeth, bones) are composed mainly of apatite Ca5(PO4)3(OH) where external shells of molluscs are mainly calcium carbonate. Silica (SiO2) is employed like a protective solid through several single-celled marine plants and within the brittle hairs of grasses and stinging nettles. Fe3O4 is employed to store iron and like it is magnetic, through 'magnetotactic' bacteria to sense the direction of the Earth's magnetic field.