Dioxygen Transport
In the human body approximately 65% of Fe is a component of hemoglobin. The protein molecule consists of four heme subunits. The 'resting state' contain high-spin Fe2+ but coordination of the strong π acceptor ligand O2 alters it to the lowspin form. It is significant for the action of hemoglobin, like the uptake of O2 through one heme sub-unit raises the O2 affinity of another, the cooperative influence making the uptake and release more proficient. High-spin Fe2+ is little too large to fit comfortably within the heme ring, although the low-spin ion is very smaller and so O2 coordination basis the Fe to shift into the ring center. The 'proximal' histidine within the Fe coordination sphere as well moves and acts like a means of communicating among sub-units. Other feature of hemoglobin is designed to lessen its affinity for another ligands. The position of the 'distal' histidine displayed in diagram 1b forces coordination in a nonlinear geometry. This is agreeable for O2 but not for strongly competing species like CO and CN-; even though these are still extremely toxic they would be even more so otherwise.
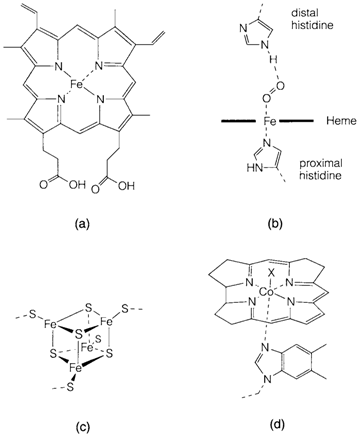
Fig. 1. Fe and Co in biomolecules: (a) heme; (b) O2 binding in hemoglobin (see text); (c) [4Fe-4S] center in iron-sulfur proteins; (d) cobalamin (B12) structure.