Dioxygen Chemistry
Absorption of sunlight in green plants through chlorophyll (that consists of magnesium;) gives energy for photosynthesis, that converts CO2 and H2O into organic compounds and dioxygen. Respiration through both plants and animals gives metabolic energy through oxidation of organic matter by using atmospheric O2. Both respiration and photosynthesis include complex electron-transfer chains, that are including redox reactions of organic (example quinones) and inorganic compounds (example Fe proteins). The terminal step within photosynthesis is
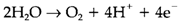
and is carried out through a unit containing four Mn ions. The O2-consuming unit in respiration (termed as cytochrome c oxidase like it acquires electrons from the heme-iron protein cytochrome c) consists of both heme-Fe and Cu at the active site. The many-electron redox step is make easy through the existence of two or more transition metals along with a number of available redox states, MnII/III/IV, CuI/II and FeII/III/IV with the ferryl (FeIV=O) state that is not general for Fe.
Intermediate oxidation states of O, peroxide (O2-2) and (O-2) superoxide are usually toxic and are undesirable side results of the exceeding reactions. Superoxide dismutase is a Cu-Zn-consisting of enzyme catalyzing the disproportionation of to O-2 and O2-2H2O; several catalases and peroxidases work to decompose peroxide.
Oxygenase enzymes catalyze particular oxidation reactions through O2. Cytochrome P-450 enzymes carry out reactions like R-H→R-OH and include a ferryl intermediate. Copper-consisting of oxygenases are usually found outside cells (than inside like with Fe; the variation is thought to reflect the later adoption of Cu in evolution) and are particularly significant in reactions that create connective tissue like collagen.