Regulation of Enzyme Activity
The Enzymes function in congregation line-like fashion to catalyze the thousands of reactions occurring in cells every second. Regulating enzymatic activities and Coordinating is essential for efficient functioning of cells. Various control mechanisms which do not include covalent changes of the enzymes are possible. Enzymatic activity is controlled at the enzyme content stages by the degradation and synthesis of enzymes as well as at the level of individual enzymatic molecules through low molecular substances (effectors) like as metabolites ADP and ATP. Whenever effectors are attached to sites other than the active centers of enzymes these sites are called as allosteric sites, and the enzymes regulated through them are known as allosteric enzymes. PFK (Phosphofructokinase) - a main enzyme in glycolysis is a typical allosteric enzyme, and is inhibited through ATP and citric acid and activated through AMP and ADM fructose 6-phosphate.
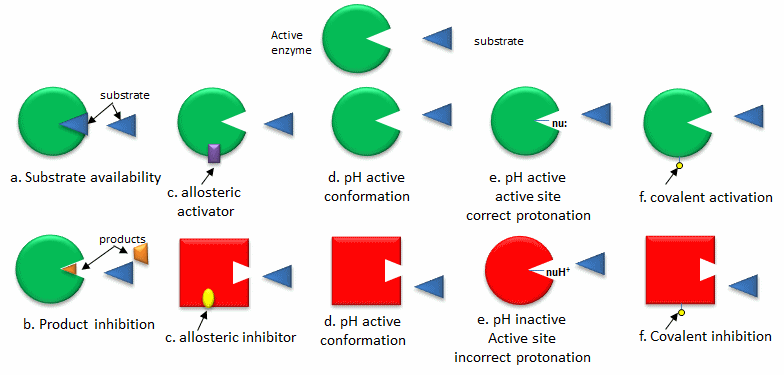
In another side from allosteric regulation enzymatic activity is also regulated by enzyme-molecule-level modification through covalent bonds. The phosphorylation included in signal transduction is a typical instance of this. In the phosphorylation of proteins a phosphoester bond is be formed in the hydroxyl group of tyrosine residue, serine or threonine.