Electron Transport and Oxidative Phosphorylation:
Most of the free energy released during the oxidation of glucose to CO2 is retained in the decreasing coenzymes NADH and FADH2 producing during glycolysis and the citric acid cycle. In During respiration, electrons are released from NADH and FADH2 and eventually are transferred to O2, creating H2O according to the reactions.
The ΔG°′ values for these tightly exergonic reactions are -52.6 kcal/mol and -43.4 kcal/mol (FADH2). Recall in which the conversion of one glucose molecule to CO2 through the glycolytic pathway and citric acid cycle yields 2 FADH2 and 10 NADH molecules. Oxidation of these reduced coenzymes has a total ΔG°′ of -613 kcal/mol. Therefore, of the potential free energy present in the chemical bonds of glucose (-680 kcal/mol), about 90 % is conserved in the decreased coenzymes.
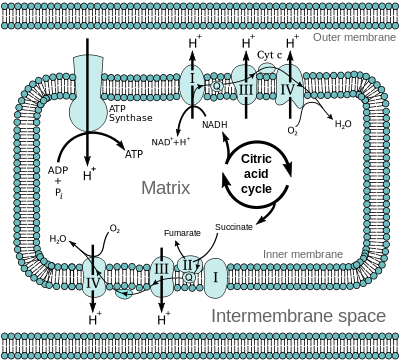
In eukaryotes, the electron transport and oxidative phosphorylation happens in the inner membrane of mitochondria. These procedures re-oxidize the FADH2 and NADH which arise from the citric acid cycle (situated in the mitochondrial matrix), glycolysis (situated in the cytoplasm) and fatty acid oxidation (situated in the mitochondrial matrix and trap the energy released as ATP. The Oxidative phosphorylation is through far the main source of ATP in the cell. In prokaryotes, the elements of electron transport and oxidative phosphorylation are situated in the plasma membrane.