Acids, Bases and pH
In a solution pH is a measure of the concentration of H. An acid is a proton donor and a base is a proton acceptor. Ionization process of an acid yields its conjugate base, and the 2 are termed a conjugate acid-base pair, for instance acetate (CH3COO) and acetic acid (CH3COOH). The pK of an acid is the pH at which it is half dissociated. The Henderson-Hasselbalch equation shows the relationship between pH, pK and the ratio of acid to base, and may be used to calculate these values.
The pH of a solution is a measure of its concentration of protons (H), and pH is explained as:
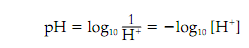
in which the square brackets mention a molar concentration.
An acid can be explained as a proton donor and a base as a proton acceptor:

For instance;
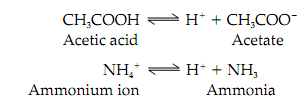
By the ionization of an acid the species formed is its conjugate base. On the other hand, protonation of a base yields its conjugate acid. So, for instance, acetate and acetic acid is a conjugate acid-base pair.
The ionization of a weak acid is following:
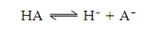
The obvious equilibrium constant (K) for this ionization is following:

The pK of an acid is following:
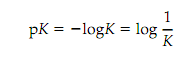
The pK of an acid is the pH at which it is half dissociated, i.e. when [A] [HA]. The Henderson-Hasselbalch equation expresses the relationship between pH and the ratio of acid to base. It is derived as given. Rescheduling of
Equation 1 gives:
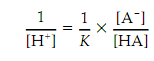
Take the logarithm of both sides of this equation gives result as:
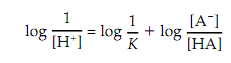
Substituting pH for log 1/[H ] and pK for log 1/K gives:
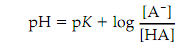
which is the Henderson-Hasselbalch equation. This equation shows that the pK of an acid is numerically equal to the pH of the solution while the molar density of the acid is equal to that of its conjugate base. From the Henderson-Hasselbalch equation the pH of a solution can be calculated if the molar concentrations of A -
and HA, and the pK of HA are known. In similar way, the pK of an acid may be calculated if the molar concentrations of A- and HA, and the pH of the solution are known already.