Ionic radii
An anion-cation distances that are experimentally measured in highly ionic solids can be interpreted on the assumption that each ion has a almost fixed radius. For instance, the variation in anion-cation distance among the halides KX and NaX is close to 36 pm irrespective of the anion X and it is general to attribute this to the variation in radii among Na+ and K+. Though, to separate the observed distances into the sum of two ionic radii is difficult to do in a completely satisfactory way. One method is to look for the minimum value in the electron density distribution among the neighboring ions, but except from the experimental problems involved such measurements do not actually support the assumption of constant radius. So, sets of ionic radii are all based ultimately on somewhat arbitrary assumptions.
Various different sets have been derived, the most extensively used being those of Shannon and Prewitt based on the assumed radius of 140 pm for O2- in six-coordination. Values for a selection of ions are displayed in Table 1.
Every consistent set of radii should be capable to give estimates of the total anioncation distance and therefore the unit cell dimensions if the structure is known. It is necessary not to mix values from different sets. Even though the values may differ, all sets show similar trends.
Table 1. Ionic radii (pm) for six-coordination, based on a value of 140 pm for O2-
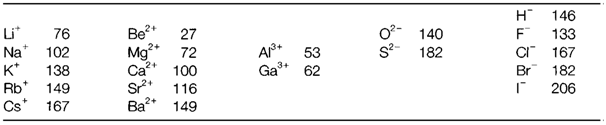
(i) Radii decrease with increasing positive charge (example Na+>Mg2+>Al3+) or decreasing negative charge (example O2->F-), for isoelectronic ions.
(ii) Radii increase down each group (example Li+<Na+< etc.).
(iii) For elements with the variable oxidation state (not displayed in Table 1) radius decreases with increasing positive charge (example Fe2+>Fe3+).
(iv) Several anions are larger than most cations.
(v) Ionic radii increase with coordination number (CN). For instance, the Shannon and Prewitt radii (in pm) for K+ with different CN (displayed in parenthesis) are: 138 (6); 151 (8); 159 (10); 160 (12).
Trends (i)-(iv) follow the changes supposed in the radii of atomic orbitals. The difference with CN is, though, very important and depicts that ions cannot be referred as hard spheres but have an effective size depending on their environment. This is supposed because the equilibrium distance among ions involves a balance of repulsive and attractive forces. Repulsive forces arrive from the overlap of closed shells and their net significance increases in proportion to the CN. Attractive forces depend on the long-range summation and are electrostatic of the interactions among many ions. Even though they increase with CN the change is much less than for short-range repulsion. Increasing the CN then changes the balance in favour of repulsive forces and leads to an increase in distance.