Voltage and Specific Gravity:
The reduction in specific gravity on discharge is proportional to the ampere-hours discharged. Although charging a lead-acid battery, the rise within specific gravity is not proportional or uniform, to the amount of ampere-hours charged that was show in the below Figure.
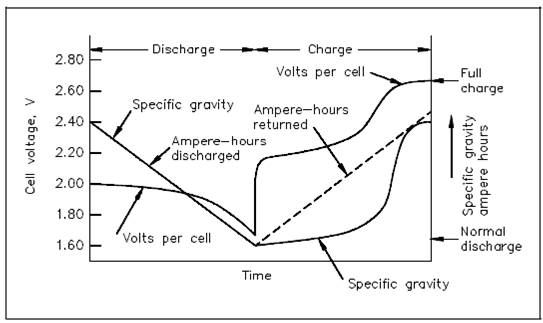
Figure Voltage and Specific Gravity During Charge and Discharge
The electrolyte in a lead-acid battery plays a direct role in the chemical reaction. The specific gravity reduces as the battery discharges and rise to its normal, real value as it is charged. Because specific gravity of a lead-acid battery reduces proportionally during discharge, the value of specific gravity at some provided time is an approximate denotes of the battery's state of charge. For determine the state of charge, compare the specific gravity, as read by using a hydrometer, along with the full charge value and the manufacturer's published specific gravity drop that is the reduction from full to nominal charge value.