Discharge and Charging of Lead-Acid Battery:
Within a lead-acid battery, two categories of lead are acted upon electro-chemically through an electrolytic solution of diluted sulfuric acid (H2SO4). A positive plate consists of lead peroxide (PbO2) and the negative plate is sponge lead (Pb), display in Figure.
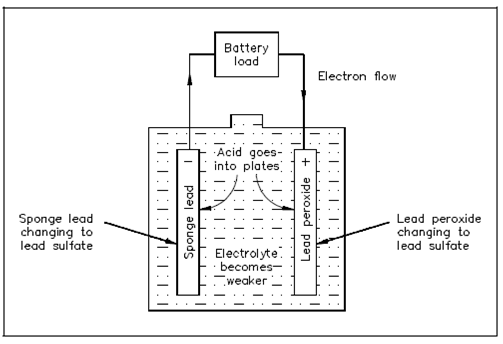
Figure Chemical Actions during Discharge
Whenever a lead-acid battery is discharged, the electrolyte divides within H2 and SO4. The H2 will combine with some of the oxygen that is formed on the positive plate to generate water (H2O), and thus decrease the amount of acid in the electrolyte. The sulfate (SO4) combines with the lead (Pb) of both plates, forming lead sulphate (PbSO4), as display in Equation (4-1).
PbO2 + Pb + 2H2SO4 → discharge 2PbSO4 + 2H2O (4-1)