Electrochemistry:
Chemicals could be merged along with certain metals to cause a chemical reaction which will transfer electrons to generate electrical energy. This procedure works on the electrochemistry principle. One instance of this principle is the voltaic chemical cell which is display in Figure. A chemical reaction generates and manages opposite charges on two dissimilar metals which serve as the positive and negative terminals. The metals are in contact within an electrolyte solution. Cells will produce a battery by Connecting together more than one of these.
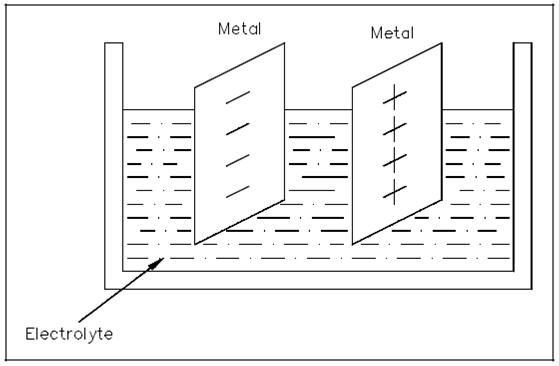
Figure: Voltaic Chemical Cell
Example: A battery could manage a potential difference among its positive and negative terminals by chemical action. Several kinds of cells and batteries will be studied in more detail in Module 4, Batteries.