Resonance structures for aniline:
Resonance effects as well describe why aromatic amines (arylamines) are weaker bases as compared to alkylamines. The lone pair of electrons on nitrogen can interact along with the π system of the aromatic ring, resultant in the probability of three zwitterionic resonance structures. (A zwitterion is a neutral molecule that consisting of a positive and a negative charge.) Because nitrogen's lone pair of electrons is included in this interaction, it is less available to create a bond to a proton and thus the amine is less basic.
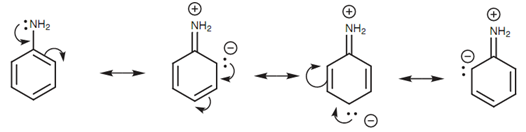
Figure: Resonance structures for aniline.