Comparison of basic strengths:
Electronegativity as well describes the order of basicity for neutral molecules like amines, alcohols, and alkyl halides.
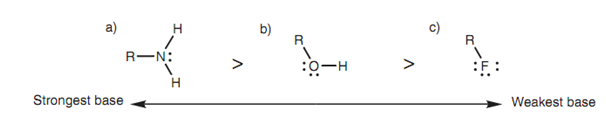
Figure: Comparison of basic strengths: (a) an amine; (b) an alcohol; (c) an alkyl ?uoride.
These neutral molecules are very much weaker bases as compared to their corresponding anions; however the order of basicity is still similar and can be described by considering the relative stability of the cations that are formed while these molecules bind a proton.
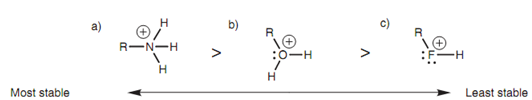
Figure: Relative stability of the cations formed from (a) an amine; (b) an alcohol; (c) an alkyl ?uoride