Amines and amides:
Amines are weak bases. They make water soluble salts in acidic solutions, and in aqueous solution they are in equilibrium along with their conjugate acid.
Amines are basic since they have a lone pair of electrons that can create a bond to a proton. Amides as well have nitrogen along with a lone pair of electrons, but not like amines they are not basic. This is since a resonance occurs within the amide structure that involves the nitrogen lone pair. The driving force after this resonance is the electronegative oxygen of the neighboring carbonyl group that is 'hungry' for electrons. The lone pair of electrons on nitrogen creates a π bond to the neighboring carbon atom. As this occurs, the π bond of the carbonyl group breaks and both electrons move onto the oxygen to provide it a total of three lone pairs and a negative charge. Because the nitrogen's lone pair is included in this resonance, it is not availabe to bind to a proton and therefore amides are not basic.
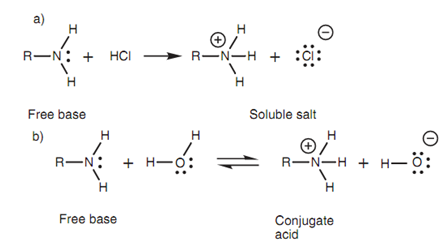
Figure: (a) Salt formation; (b) acid-base equilibrium.
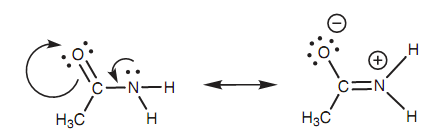
Figure: Resonance interaction of an amide.