Acid–base equilibrium of methylamine and water:
pKb
It is a measure of basic strength. If methylamine is dissolved in water, equilibrium is set up (Fig. 5).
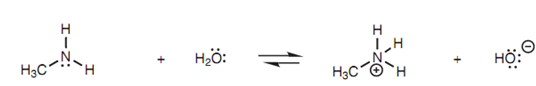
Figure: Acid-base equilibrium of methylamine and water.
Methylamine on the left side of the equation is known as the free base, whereas the methyl ammonium ion formed on the right side is known as the conjugate acid. The extent of ionization or dissociation in the equilibrium reaction is described by the equilibrium constant (Keq);
Keq = [Products]/ [reactants] = [CH3NH3+][HO-]/ [CH3NH2][H2O]
Kb = Keq [H2O] = [CH3NH3+] [HO-]/ [CH3NH2]
Keq is generally measured in a dilute aqueous solution of the base and thus the concentration of water is high and supposed to be constant. Hence, we can rewrite the equilibrium equation in a much simple form in which Kb is the basicity constant and involves the concentration of pure water (55.5 M). pKb is the negative logarithm of Kb and is employed like a measure of basic strength (pKb = Log10Kb).
A large pKb points out a weak base. For instance, the pKb values of ammonia and methylamine are 4.74 and 3.36, correspondingly that points out that ammonia is a weaker base as compared to the methylamine.
pKb and pKa are related via the equation pKa+ pKb = 14. Therefore, if one knows the pKa of an acid, the pKb of the conjugate base can be calculated and conversely.