Flame atomiser:
You have learnt about flame atomisers. In a classic flame atomisation process, the analyte solutions are commonly nebulised along with the help of a nebuliser into a spray chamber. An aerosol so generates along with a mixture of a burning gas and an oxidant is directed into a suitable burner. Flame temperature depends on fuel-oxidant ratio and the requisite temperature for analysis could be acquired through varying the fuel-oxidant ratio. The fuel- oxidant combinations generally used in AAS, the corresponding combustion reactions and a flame temperature are given within Table.
Table: The fuel-oxidant combinations commonly used in AAS, the corresponding chemical reaction and the corresponding flame temperatures
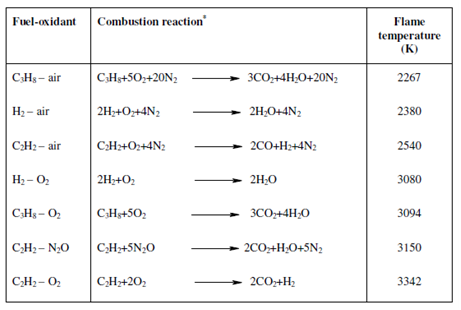
*N2 is involved in air just to show its stoichiometry.
Although analysing liquid samples, flame is considered to be superior within terms of performance features and reproducible behaviour though sampling efficiency and sensitivity of other techniques are better. This is since large amount of the sample flows down the drain and the residence time of individual atoms within the path length of flame is of the sequence of ~0.1ms. The region of maximum absorption is restricted to exact areas of the flame.
Concentration of atoms might vary hugely if the flame is moved associative to the light path either vertically or laterally from the resonance line source. A position of observation within the flame and the fuel-oxidant ratio must be correctly optimised for every element in AAS. A fuel-oxidant ratio and observation heights are so chosen as to gives the maximum number of free atoms although minimising interferences from emission, ionisation or compound creation.