Orbitals and shells:
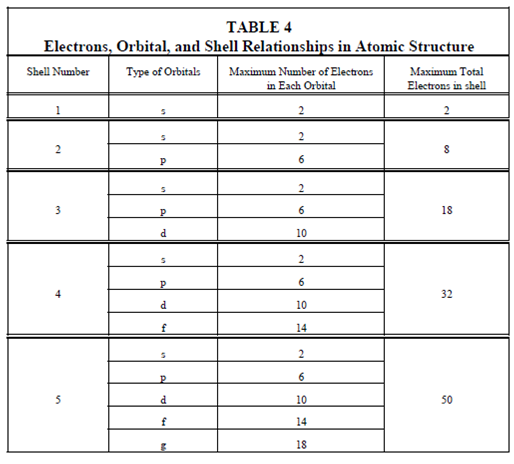
A numbers of electrons which could occupy the various orbitals and shells are displays in Table.
A more specific statement could now be made about that electrons are included within chemical reactions. Chemical reactions include primarily the electrons in the outermost shell of an atom. A word outermost shell refers to the shell farthest from the nucleus which has a few or all of its allotted number of electrons. A few atoms have more than one partially-filled shell. Whole of the partially-filled shells have a few effect on chemical behavior, other than the outermost one has the greatest effect. The outermost shell is known as the valence shell and the electrons in which shell are known as valence electrons. The word valence (of an atom) is described as the number of electrons an element loses or gains, or the number of pairs of electrons it shares while it interacts along with other parts.
The periodic chart is arranged so in which the valence of an atom could be simply determined. For the components in the A groups of the periodic chart, a number of valence electrons is the similar as the group number; that is, carbon (C) is in Group IVA and has there four valence electrons. Noble gases (Group 0) have eight in their valence shell along with the exception of helium that has two.