Atomic orbitals:
Carbon comprises six electrons and is in row 2 of the periodic table. The meaning of this is that there are two shells of atomic orbitals that are available for these electrons. The ?rst shell closest to the nucleus comprises only one s orbital - the 1s orbital. The second shell has only one s orbital (the 2s orbital) and three p orbitals (3 * 2p). Hence, there are a total of ?ve atomic orbitals in which these six electrons can be ?t. The s orbitals are spherical in shape along with the 2s orbital being much larger as compared to the 1s orbital. The p orbitals are dumbbell-shaped and are aligned together the x, y and z axes. Hence, they are assigned 2px, 2py and 2pz atomic orbitals.
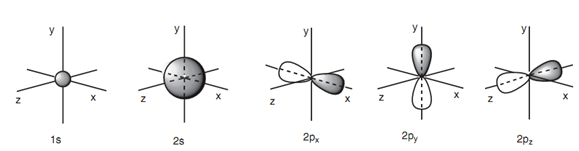
The atomic orbitals explained above are not of equivalent energy. The 1s orbital comprises the lowest energy. The 2s orbital is come next in energy and the 2p orbitals have the highest energies. The three 2p orbitals have similar energy, meaning which they are degenerate.
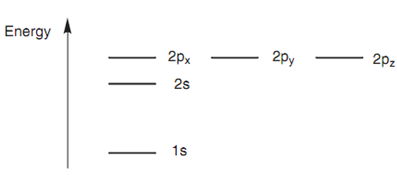
Figure: Energy levels of atomic orbitals.