Hydrogenic ions
The correct solutions of Schrödinger's equation can be applied to hydrogenic ions with one electron: instnaces are He+ and Li2+. Orbital energies and sizes now depend on the atomic number Z, that is equal to the number of protons in the nucleus. Average radius <r> of an orbital is
<r>≈ n2 a0/ Z
In hydrogen, where a0 is the Bohr radius (59 pm) the average radius of a 1s orbital. So electron distributions are pulled in in the direction of the nucleus by the increased electrostatic attraction with higher Z. The energy (see Equation 1) is
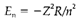
The issue Z2 arises because the electron-nuclear attraction at a given distance has increased by Z and average distance has also decreased by Z. So the ionization energy of He+ (Z=2) is four times that of H and that of Li2+ (Z=3) nine times.