Angular functions: 'shapes'
The mathematical functions for atomic orbitals might be written as a result of two factors: the radial wave function explains the behaviour of the electron as a function of distance from the nucleus; the angular wave function depicts how it changes with the direction in space. Angular wave functions not depend on n and are characteristic features of the s, p, d,...orbitals.
Table 1. Atomic orbitals with n=1-4
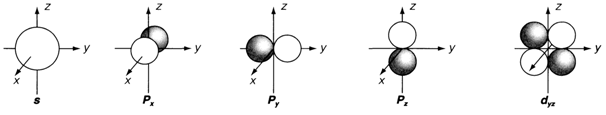
Diagram 1. The shapes of s, p and d orbitals. Shading depicts negative values of the wave function. More d orbitals are displayed in.
Pictorial representations of angular functions for s, p and d orbitals are shown in diagram. 1. Mathematically, they are essentially polar diagrams depicting how the angular wave function depends on the polar angles θ. informally, they can be considered as boundary surfaces enclosing the region(s) of space where the electron is most probably to be found. An s orbital is represented by a sphere, since the wave function does not depend on angle so that the possibility is similar for all directions in space. Each p orbital has two lobes, with the positive and negative values of the wave function either side of the nucleus, separated by a nodal plane where the wave function is zero. The three separate p orbitals subsequent to the allowed values of m are directed along distinct axes, and sometimes denoted as px, py and pz. The five dissimilar d orbitals (one of which is displayed in diagram. 1) each have two nodal planes, separating two negative and two positive regions of wave function. The f orbitals (not shown) each have three nodal planes.
The shapes of atomic orbitals displayed in diagram. 1 is significant in understanding the bonding properties of atoms.