Spectrum of 100 pm cerium:
Depending on the composition of the analyte, the excited species consist of atoms, singly charged ions and sometimes the doubly charged ions. The emission lines observed are mainly from the excited atoms and singly charged ions; the emissions from the doubly charged ions however are relatively rare. The energies of the transitions are such that the emitted radiation falls in the ultraviolet and visible region of the spectrum i.e. among 160-900 nm. The emission lines from the atomic and ionic species are very narrow; the width being less than 5 pm.
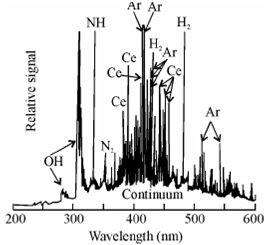
Figure: ICP-AES spectrum of 100 pm cerium
A representative atomic emission spectrum based on plasma is given in Figure; it shows an ICP-AES spectrum of cerium having a concentration of 100 pico mol. You may note the presence of a number of lines from the analyte, the source gas (Ar), and the molecular species and other background radiation. This would give you an idea of what to expect for a multielement analyte.