Atomic Displacements
If a goal or struck nucleus gains about 25 eV of kinetic energy (25 eV to 30 eV for most metals) within a collision along with a radiation particle (commonly a fast neutron), the nucleus will be displaced from its equilibrium position in the crystal lattice, as display in below figure.
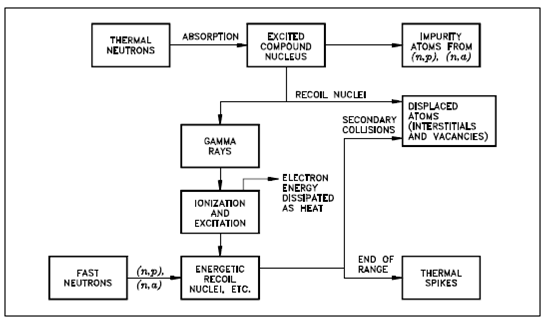
Figure: Thermal and Fast Neutrons Interactions with a Solid
The goal nucleus (or recoiling atom) which is displaced is known as a knocked-on nucleus or just a knock -on (or primary knock-on).
While a metal atom is ejected from its crystal lattice the vacated site is known as a vacancy .The amount of energy required to displace an atom is known as displacement energy. An ejected atom will travel by the lattice causing heating and ionization. If the energy of the knock-on atom is large sufficient, it might in turn generates additional collisions and knock-ons. Those knock-ons are referred to as secondary knock-ons. The procedures will continue until the displaced atom does not have enough energy to eject another atom from the crystal lattice.Thus, a cascade of knock-on atoms will increase from the initial interaction of a high energy radiation particle along with an atom in a solid.
This effect is especially important when the knock-on atom (or nucleus) is generates as the output of an elastic collision along with a fast neutron (or another energetic heavy particle). The energy of the main knock-on could then be quite high, and the cascade might be extensive. A single fast neutron in the greater than or equal to 1 MeV range could displace a few thousand atoms. Many of these displacements are temporary.At high temperatures, the number of permanently displaced atoms is smaller than the initial displacement.
In During a lengthy irradiation (for huge values of the neutron fluence), several of the displaced atoms will return to normal (stable) lattice sites (which is, partial annealing occurs spontaneously). The permanently displaced atoms might lose their energy and engage positions other than normal crystal lattice sites (or nonequilibrium sites), therefore becoming interstitials.The presence of interstitials and vacancies makes it harder for dislocations to move by the lattice. That increases the strength and decrease the ductility of a material.
At high energies, the main knock-on (ion) will lose energy primarily through ionization and excitation interactions as it passes by the lattice, as shown in Figure 3. As the knock-on loses energy, it tends to pick up free electrons which efficiently reduce its charge. Conclusion, the principle mechanism for energy losses progressively changes from one of ionization and excitation at high energies to one of elastic collisions which generates secondary knock-ons or displacements. Commonly, most elastic collisions among a knock-on and a nucleus occur at low kinetic energies below A keV, where A is the mass number of the knock-on.If the kinetic energy is greater than A keV, the probability is in which the knock-on will lose much of its energy in causing ionization.