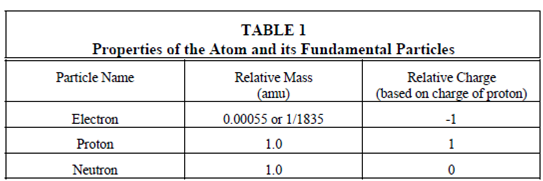
Properties of the atom:
The particles which orbit the nucleus are electrons. They are extremely small, along with a mass just 1/1835 the mass of a proton or neutron. Every electron is negatively charged, the charge of one electron is equal in magnitude (but opposite in sign) to the charge of one proton. A number of electrons orbiting a nucleus are exactly equal to the number of protons contained in which nucleus. The equal and opposite charges cancel each another, the atom as a whole is neutral. An electron is bound in the atom through electrostatic attraction. The atom remains neutral unless a few external forces cause a modification within the number of electrons.
The diameter of the atom is determined through the range of the electrons in their travels around the nucleus and is around 10-8 cm. The diameter of the nucleus is approximately 10,000 times smaller, about 10-13 to 10-12 cm. Since the nucleus is composed of neutrons and protons which are about 1835 times heavier than an electron and the nucleus contains practically all the mass of the atom, but constitutes a very small fraction of the volume. Although electrons are individually extremely small, the space in that they orbit the nucleus constitutes the major part of the atomic volume. Figure describes these size relationships, other than not to scale. The electrons would be various hundred feet away if the nucleus were the size displays.
A few of the properties of the atom and its elements parts are summarized in Table 1. A mass listed in Table 1 are measured in atomic mass units (amu) that is a associative scale in that the mass of a proton is about 1.0.