Dichlorobenzene:
Along with disubstituted aromatic rings, the position of substituents have to be set by numbering around the ring like that the substituents are located at the lowest numbers possible, for instance, the structure in the below diagram is 1,3-dichlorobenzene and not 1,5-dichlorobenzene.

Figure: 1,3-Dichlorobenzene
On the other hand, the words ortho, meta, and para can be employed. These words describes the relative position of one substituent to other substituent. So, 1, 3- dichlorobenzene can also be termed as meta-dichlorobenzene. This can be abbreviated to m-dichlorobenzene. The instances in below diagram demonstrate how different parent names may be employed.
Note: The substituent that defines the parent name is defined as position 1. For instance, if the parent name is toluene, the methyl group has to be at position 1.
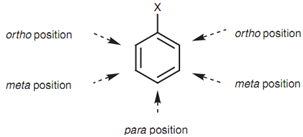
Figure: ortho, Meta and Para positions of an aromatic ring.
While more than two substituents are available on the aromatic ring, the ortho, meta, para nomenclature is no longer applicable and numbering has to be employed. Again, the appropriate substituent has to be located at position 1 if the parent name is toluene, aniline, et cetera.
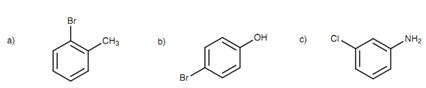
Figure: (a) 2-Bromotoluene or o-bromotoluene; (b) 4-bromophenol or p-bromophenol; (c) 3-chloroaniline or m- chloroaniline.
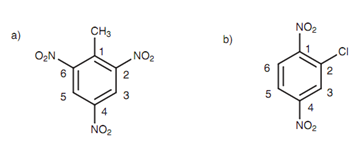
Figure: (a) 2, 4, 6-Trinitrotoluene; (b) 2-chloro-1, 4-dinitrobenzene.
The numbering is selected like that the lowest possible numbers are employed, if the parent name is benzene. In the instance shown, any other numbering would result in the substituents comprising higher numbers.
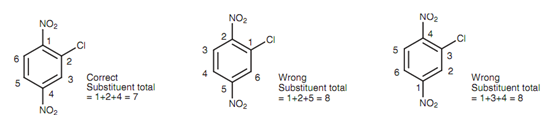
Figure: Possible numbering systems of tri-substituted aromatic ring.