Bonding molecular orbital for benzene:
Actually, the C-C bonds in benzene are all similar length. In order to identify this, we require looking more closely at the bonding that takes place. Figure displays benzene with all its σ bonds and is drawn like that we are looking into the plane of the benzene ring. Because all the carbons are sp2 hybridized, there is a 2py orbital left over on each carbon that can overlap with a 2py orbital on either side of it. By this, it is clear that every 2py orbital can overlap along with its neighbor's right round the ring. This directs to a molecular orbital that involves all the 2py orbitals in which the upper and lower lobes merge to provide two doughnut-like lobes above and below the plane of the ring. The molecular orbital is symmetrical and the six π electrons are supposed to be delocalized around the aromatic ring because they are not localized among any two particular carbon atoms. The aromatic ring is frequently presented as displayed in diagram to present this delocalization of the π electrons.
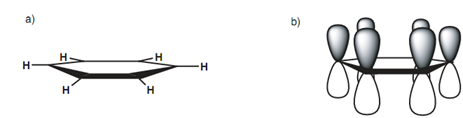
Figure: (a) σ bonding diagram for benzene, (b) π bonding diagram for benzene.
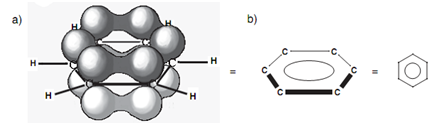
Figure: Bonding molecular orbital for benzene; (b) representation of benzene to illustrate delocalization.
Delocalization raises the stability of aromatic rings like that they are less reactive than alkenes (that is it needs more energy to disrupt the delocalized π system of an aromatic ring as compared to it does to break the isolated π bond of an alkene).