Synthesis of Di- and Tri- Substituted Benzenes:
Di substituted benzenes
A complete understanding of how substituents direct further substitution is important in planning the synthesis of a di substituted aromatic compound. For instance, there are two choices that can be made in attempting the synthesis of p-bromonitrobenzene from benzene. We could brominate first after that nitrate, or nitrate first and after that brominate. Learning of how substituents influence electrophilic substitution permits us to select the most appropriate route.
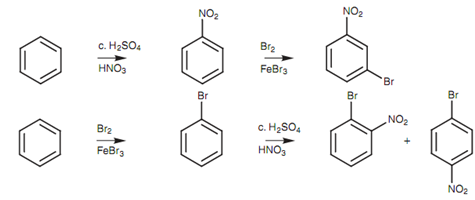
Figure: Synthetic planning to di-substituted benzenes.
In the first technique, nitrating first after that brominating would provide predominantly the Meta isomer of the final product because of the Meta directing properties of the nitro group. The second method is better because the directing properties of bromine are in our favor. Actually, we would have to separate the para product from the ortho product, although we would still get a higher yield through this route.