Sulfonation and nitration:
Sulfonation and nitration are electrophilic substitutions that involve strong electrophiles and do not require the presence of a Lewis acid as shown in figure.
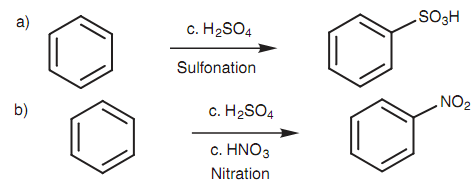
Figure: (a) Sulfonation of benzene; (b) nitration of benzene.
In sulfonation, the electrophile is sulfur trioxide (SO3) that is generated within the acidic reaction conditions.
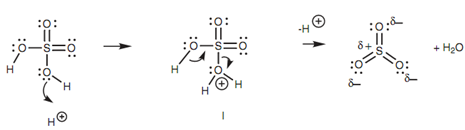
Figure: Generation of sulfur trioxide.