Oxidation and Reduction:
Oxidation
Aromatic rings are considerably stable to oxidation and are resistant to oxidizing agents like potassium permanganate or sodium dichromate. Though, alkyl substituents on aromatic ring are unexpectedly susceptible to oxidation. This can be put to good utilize in the synthesis of aromatic compounds because it is feasible to oxidize an alkyl chain to a carboxylic acid with no oxidizing the aromatic ring. The mechanism of this reaction is not completely understood, but it is known that benzylic hydrogen has to be present (that is the carbon directly attached to the ring must comprise hydrogen). Alkyl groups lacking benzylic hydrogen are not oxidized.
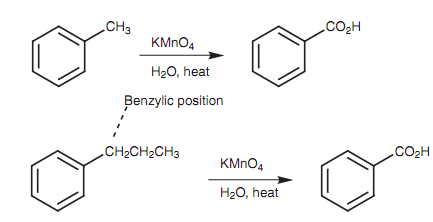
Figure: Oxidation of alkyl side chains to aromatic carboxylic acids.