Hückel rule:
An aromatic molecule have to be cyclic and planar with sp2 hybridized atoms (that is conjugated), but it must as well obey what is termed as the Hückel rule. This rule says that the ring system must comprises 4n + 2 π electrons in which n = 1, 2, 3, etc. hence; ring systems that have 6, 10, 14 ... π electrons are aromatic. Benzene fits the Hückel rule because it has six π electrons. Cyclooctatetraene has eight π electrons and does not follow the Hückel rule. Even though all the carbon atoms in the ring are sp2 hybridized, cyclooctatetraene reacts such as a conjugated alkene. It is not planar, the π electrons are not delocalized and the molecule contains alternating single and double bonds. Though, the 18-membered cyclic system does fit the Hückel rule (n= 4) and is a planar molecule along with aromatic properties and a delocalized π system.
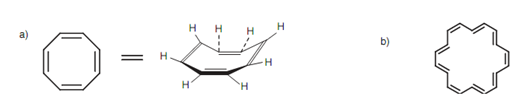
Figure: (a) Cyclooctatetraene; (b) 18-membered aromatic ring.
It is as well probable to get aromatic ions. The cyclopentadienyl anion and the cycloheptatrienyl cation are both aromatic.
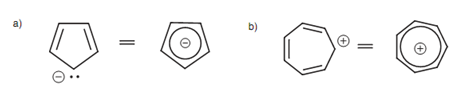
Figure: (a) Cyclopentadienyl anion; (b) cycloheptatrienyl cation.
Both of them are cyclic and planar, consisting of six π electrons, and all the atoms in the ring are sp2 hybridized.
Bicyclic and polycyclic systems can as well be aromatic.
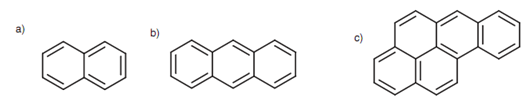
Figure: (a) Naphthalene; (b) anthracene; (c) benzo[a]pyrene.