Separation of Actinide Elements:
Similar to the separation of lanthanides ion exchange chromatography has played a major role in the separation of actinides, especially trans plutonium elements. Glen T. Seaborg used ion exchange equipment to identify each element of the 5f series beyond any doubt by the sequence of their appearance analogues to the sequence of the corresponding 4f elements. The primary valency of all the actinides is +3 similar to the lanthanides which also exhibit decreasing ionic radii (cf lanthanide contraction). In practice, solution containing all the actinides is sorbed on the top layer of a column containing acidic cation exchanger. Individual actinides are eluted from the resin bed by passage of an eluent solution through the column of resin as shown in Figure.
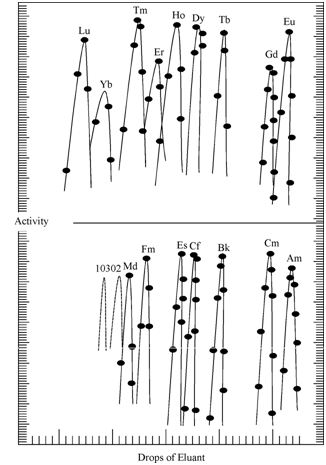
Figure: Elution of tripositive actinide and lanthanide ions
The elution is accomplished by the use of another metal ion (i.e. M3+) which shifts the equilibrium of the exchange reaction through competition with M+ for position in the resin. Alternatively, it is also achieved by adding a complexing anion to the solution which, by reducing the concentration of free metal ions, also shifts the equilibrium to the left. In Figure is shown the elution sequence for the separation of the lanthanide and actinide cations from a column of cation exchange resin using a complexing agent. It was considered as the big triumph for ion exchange chromatography for the separation of various actinides which was otherwise considered as impossible.