Oxidation of organic materials:
Oxidation of organic materials if present masks the thermal effect of substance. Thus the analysis of like cases should preferentially be carried out in inert atmosphere or within vacuum. Organic material sometimes be erasable through a appropriate solvent or might be oxidized through treating with H2O2. While clays or ore holds organic material then a huge exothermic peak appears among 200 - 600 0C. Therefore the appearance of a broad exothermic peak among 200-600 0C denotes the presence of organic material within a given sample.
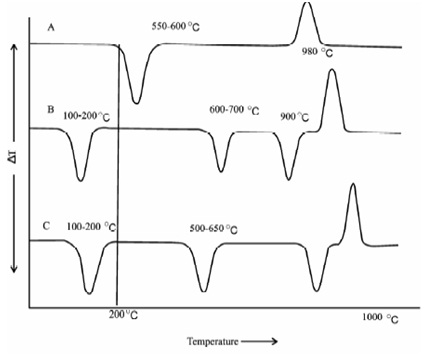
In practice therefore, difficulties do arise in a case while peak areas for two distinct samples of similar mineral provide peaks at two different temperatures. It is, thus, essential for qualitative work to know the peak area given through pure mineral identical to which in the sample under investigation. This, therefore, is not possible. Another limitation in the quantitative work within the occurrence of overlapping of peaks e.g. while Kaolinite and Illite are present in the similar sample almost completely overlapping takes place. A representative DTA curve of a few minerals is shown in Figure. It might be observed which none of these miners display an exothermic peak in the range 200- 600 °C corresponding to organic material as described above.