Ion Selective Membrane Electrode:
The analytical applications of membranes are largely in the area of ion selective membrane electrodes and particulate analysis. For most of the ion selective membrane electrodes, the role of membranes is not to transport specific ions but to selectively adsorb on either side giving rise to measurable electrical potential difference. A particular membrane permits only a particular kind of ion to penetrate and adsorb. Ion selective membrane electrodes have many applications in water analysis and environmental monitoring. The principle of operation of membrane electrode is given in Figure.
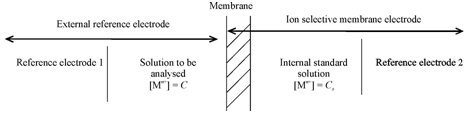
Figure: Basic principle of ion selective membrane electrode for metal ion determination
The electrical potential developed across the membrane, measured using ion selective membrane electrode and reference electrode allows the determination of metal ion as per Nerst equation given below.
E = 2.303 RT / n F log C/CS
In principle, all factors associated with the use of the electrode, other than the ion concentration to be measured, remain constant and the measurement of the cell potential is directly proportional to the logarithm of ion concentration.