Mixture of calcium and magnesium oxalates:
Calcium oxalate monohydrate is unsatisfactory weighing form for determining calcium as oxalate because its tendency to retain excess moisture even at 110 °C, co-precipitated ammonium oxalate leftovers un-decomposed. A anhydrous calcium oxalate is hygroscopic other than CaCO3 is excellent weighing form if heated to 500 ± 25 °C as can be seen in TG curve of CaC2O4.H2O. Above 635 0C the decomposition of CaCO3 commences to become significant under usual laboratory conditions and completely converted to CaO at 900 °C. Therefore, calcium oxalate monohydrate decomposes within three steps, first dehydration, second removal of CO and formation of CaCO3 then third step CaCO3 decomposes to CaO.
CaC2O4.H2O → CaC2O4 + H2O at about 135-220 °C
CaC2O4 → CaCO3+CO at about 400-480 °C
CaCO3 →CaO+ CO2 at about 635-900 °C
But magnesium oxalate dihydrate decomposes in two steps alternatively of three steps. Initial, dehydration then removal of CO and CO2 concurrently and forming MgO, there is no horizontal corresponding to MgCO3 as it is thermally unstable at this temperature.
MgC2O4.2H2O → MgC2O4+2H2O at about 135-220 °C
MgC2O4 → MgCO3+CO→MgO+CO2 at about 500 °C
The TG curve for the combination of CaC2O4.H2O and MgC2O4.2H2O display two mass losses up to 500 °C, first at about 200 °C because of loss of water and the second mass loss occurs in the 390-500 °C range that is because of decomposition of both calcium and magnesium oxalates. Therefore, at 500°C, the composition of the combination will be CaCO3 and MgO. Third mass loss after 500 °C is because of only the decomposition of CaCO3. If m1 and m2 are the mass of the combination at 500 °C and 900 °C then same to the previous instance we could calculate the amount of calcium and magnesium in the original sample.
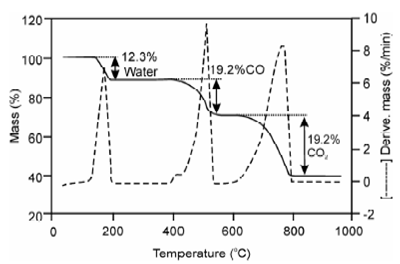
Figure: DTG/TG curve of calcium oxalate