Complex mixture:
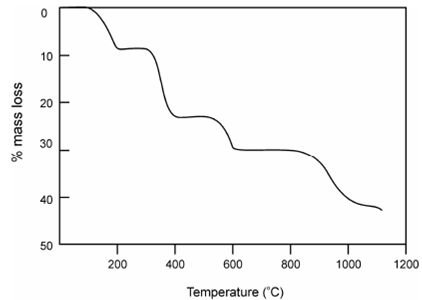
Within this category we are considering the analysis of a mixture of calcium, barium and strontium oxalates. In the thermogram of a mixture of Ca, Sr and Ba oxalates it is remember that in among 100 °and 250 ° C the water of hydration is evolved from all metal oxalates, the curve exhibited a horizontal weight level from 250-360 °C after the loss of the water of hydration that corresponds to the composition for anhydrous metal oxalates. After that all the three oxalates decomposed concurrently to the carbonates and the procedure finished at 500°C.Then from 500 °C to 620 °C the anhydrous carbonates were stable. On additional heating, the CaCO3 decomposed in the temperature range 620°C- 860 °C to oxide followed through the decomposition of SrCO3 from 860 °C to 1100°C at that temperature BaCO3 began to decompose. From weight loss curve, the subsequent data are acquired.
I- mass of hydrated oxalates at 100 °C (mass of the sample) = ms
II- mass of water of hydration = m1
III- mass of CO formed through the decomposition of metal oxalates = m2
IV- mass of CO2 formed through the decomposition of CaCO3 = m3
V- mass of CO2 formed through the decomposition of SrCO3 = m4