Analysis of a mixture of AgNO3 and Cu (NO3)2:
The TG curve of pure AgNO3, Cu (NO3)2 and their combination are given in Figure. Curve a is the curve of dry crystalline AgNO3. A horizontal extends to 340 °C and is followed through descent as far as 473 °C. At this temperature decomposition sets in abruptly and nitrous fumes are expelled up to 610 °C. After in which there is much slower mass loss from 610 °C to 810 °C.
Decomposition of AgNO2, which is not observed while CuO is present, no doubt the latter catalyses the decomposition. Above 810 °C the weight is again constant because of the creation of pure Ag metal.
2AgNO3(s) → 2AgNO2 (s) + O2 (g)
2AgNO2(s) → 2Ag(s) + 2NO2 (g)
Although the copper nitrate hexahydrate gives a quite dissimilar curve b. Water and nitrogen oxide are driven off up to 150 °C to 200 °C, after that a horizontal denotes the existence of zone of a new compound, which has been analyzed and it corresponds to a basic nitrate Cu(NO3)2 2Cu(OH)2. After that among 200 °C and 610 °C this compound decomposes vigorously from 250 °C and consequently more slowly. The residue is CuO that only become constant at 940 °C.
3Cu (NO3).6H2O → Cu (NO3)2.2Cu (OH)2 +H2O + NO → CuO
If a combination of AgNO3 and Cu (NO3)2 is placed in thermobalance, a curve c is recorded. The horizontal associated to the basic copper nitrate is, nevertheless, well marked. From 240 °C to 400 °C there is a residue keeping constant mass (AgNO3 + CuO), although above 900 °C a combination of Ag + CuO is present.
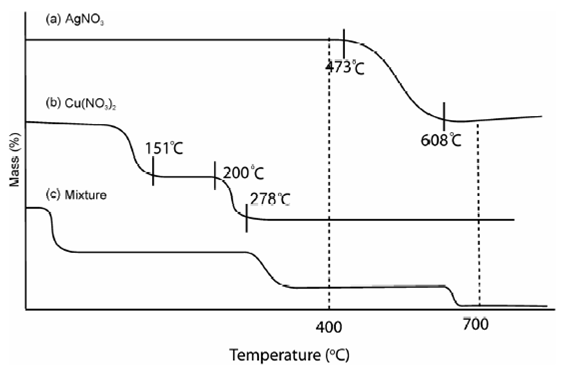
Figure: TG curve of nitrates: A: AgNO3, B: Cu(NO3)2 and C: AgNO3 + Cu(NO3)2 mixture
If m1 and m2 are the mass of the sample at 400 °C and 700 °C, correspondingly, the amount of copper and silver in the sample could be calculated same to previous example.
A binary alloy of Ag and Cu could be analyzed with ± 3 % error through this method through dissolving the alloy in HNO3 and then running thermogram and recording successive weight at 400 °C and 700 °C through solving subsequent concurrent equations:
(170/108) x + (79/63) y = m1
x + (79/63) y = m2
Here in the above equation x and y are the masses of Ag and Cu in the alloy, m1 and m2 are the masses of the sample at 400 °C and 700 °C, correspondingly, 170 is the molar mass of AgNO3, 1000 is the associative atomic mass of Ag, 79 is molar mass of CuO and 63 is the relative atomic mass of Cu.