Analysis of a mixture of calcium and magnesium carbonates:
A typical TG curve of a mixture of calcium and magnesium carbonates is display in Figure. You can notice that a significant mass loss occurs before 210° C. This is due to the moisture present in the mixture. Another mass loss at about 480° C is because of the following reaction:
MgCO 3 (s) → MgO (s) + CO2 (g)
Previously we mentioned that CaCO3 decomposes at about 800 °C. In Figure mass loss between about 600 °C and 900 °C can be interpreted because of decomposition of CaCO3.
CaCO3 (s) → CaO (s) + CO2 (g)
Portion of the curve ab represents a mixture of MgO and CaCO3 and cd represents a residue of the mixture of MgO and CaO. Both these plateaus ab and cd represent weight m1 and m2, respectively. In fact mass m1 is due to CaCO3 + MgO.
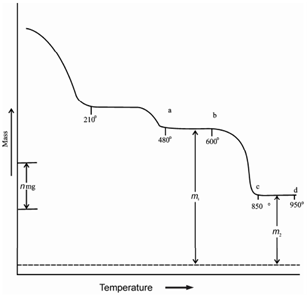
Figure: TG curve of mixture of calcium and magnesium carbonate
Thus, m1 - m2 is the loss of CO2 between 500° C and 900° C due to the decomposition of CaCO3. Using TGA curve we could associate the mass of different components formed during TGA experiment.