Substoichiometric Isotope Dilution Analysis (SIDA):
A variation in isotope dilution analysis method was suggested through J. Ruzicka and J. Stary in 1963. A necessity to determine specific activity makes IDA a hard method for trace determination because it is essential to isolate sufficient weighable amount of substance. That difficulty is overcome in substoichiometric IDA where a standard with known amount of analyte and the unknown solution are subjected to an identical separation process. In this procedure similar amount of reagent is added that is a little less than which needs soichiometrically (substoichiometric) in both standard and the unknown solutions so in which similar amount of analyte is extracted in both cases. Ideally an amount of radiotracer added to the unknown should be approximately of the similar order. A described of the principle of SIDA is displays in Figure
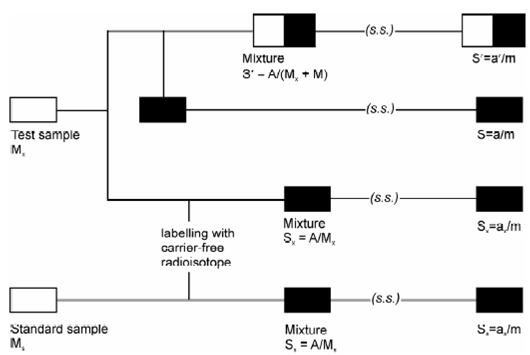
Figure: Illustration of principle of substoichiometric isotope dilution analysis