Applications Of Amperometric Titrations
Amperometric titrations have even huger range of applications than polarography since even electro-inactive substances could be determined using electro-active titrant.
According to Ilkovic equation id is proportional to concentration keeping all other factors of the equation constant. Then, if a few of the electroactive material in the solution is removed through interaction along with some other reagent (e.g.: EDTA reagent for Zn2+ determination) the diffusion current will decrease. This is the fundamental principle of polarographic titrations or amperometric titrations.
The diffusion current at a suitable applied voltage is measured as a function of the volume of the titrating solution. The last point is the intersection of two lines giving the change of current before and after the equivalence point.
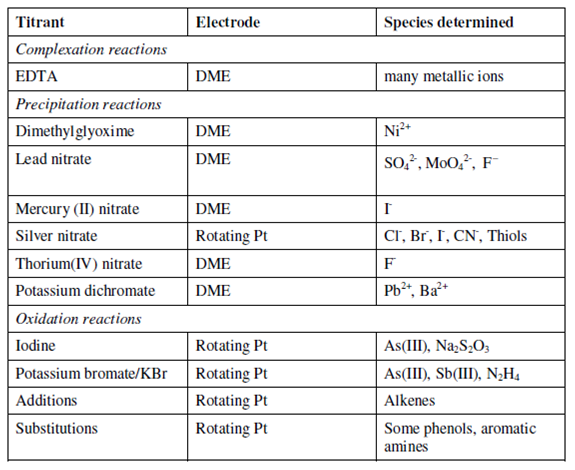
Table: Examples of amperometric titrations