Analysis of Elements by Phosphorescence Spectroscopy:
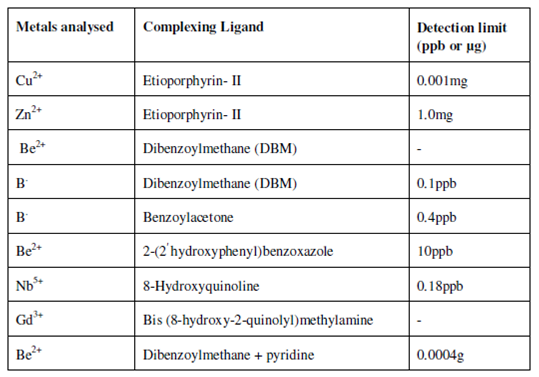
A number of ionic compounds also exhibit intense room temperature phosphorescence. Various metal ions such as transition elements (Cu, Zn, Nb, Gd) as well as s-block elements (Be) have been analysed through phosphorimetry. In addition, those elements that cause fluorescence quenching (e.g, Fe, Cu, Co, Ni, Cr) can be analysed through phosphorimetry, therefore, these requires to be analysed as coordination complexes. A complexing ligands used are ββ- dketones like dibenzoylmethane (DBM) benzoylacetone or quinoline derivatives like 8-hydroxyquinoline, etc. It is probable to analyse metals at extremely low concentration e.g., (Cu 0.001mg) Be (0.0004 µ g) of the element can be analysed.
It is also possible to determine metals in the mixtures. A Beryllium complex win HDBM as Be (DBM)2 is extracted in carbon tetrachloride and is separated from group of transition and representative elements thereby avoiding the step of separations to eliminate interferences. Amongst non metals, boron is efficiently analysed from marine environment, so also Nb (V) was extracted in oxine at pH 9.4 with chloroform as the solvent. Be while complexed along with DGM and pyridine is measured at 527 nm.
Table: Complexing ligands for the determination of metal ions and the detection limit of the methods
The rare earths and uranyl components phosphoresce and a number of them, particularly terbium and europium, are used as phosphors in lamps and TV tubes. A phosphorescence intensity of the rare earths rises tremendously while they are covalently bound to certain molecules and this feature has been used in the analysis of transferin within blood.