Qualitative Applications:
Literature is full of references illustrating identification of organic compounds and their structure determination by NMR spectroscopy. We shall discuss the spectral characteristics of some typical examples of simple organic compounds. We have chosen examples of aliphatic and aromatic compounds containing a hydroxyl group and would like to demonstrate some structural and dynamic features that may be studied by NMR. We start along with a simple molecule, ethanol.
Ethanol is a classical example of identification, spin-spin coupling and structure determination including exchange phenomenon of an organic compound. A low resolution NMR spectrum of ethyl alcohol consists of three broad peaks corresponding to three chemically different types of protons with peak areas in the ratio of 3:2:1. That suggests for the presence of three protons of one type, two of another type and one of third type which correspond to methyl (-CH3), methylene (-CH2) and an alcoholic (-OH) group correspondingly. Of these, -OH proton is observed at most downfield because it is attached to an electronegative oxygen atom and the one corresponding to methyl group is observed high field and has peak area three times that of hydroxyl proton.
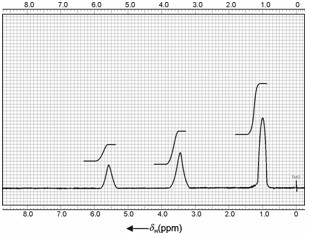
Figure: Low resolution NMR spectrum of pure ethanol, CH3-CH2-OH