Fragmentation by Cleavage at a Single Bond
A simple cleavage of the single bond in the molecule is quite common and important fragmentation mechanism. Inside such a cleavage of a radical cation we get a radical and a cation. For example, the simple cleavage of C-C single bond in molecular ion radical of propane can be represented as follows.
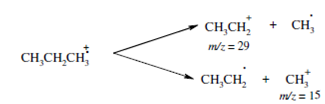
In this cleavage we get two cations with the m/z values of 29 and 15 respectively and both of them are observed in the mass spectrum of propane. However, the intensity of the peak at m/ z 29 is much more than that of the peak at m/z 15. In fact the m/z 29 peak is the base peak which is the most intense peak. The intensities of the peaks is determined by the stability of the fragment ions; the secondary ethyl carbocation (CH3CH2 +) is much more stable than the primary methyl carbocation (CH3 +).
For the similar purpose the cleavage is favoured at more alkyl substituted carbons in branched alkanes.