Monitoring the Progress of a Reaction
The other useful application of IR spectrometry is to monitor the progress of a reaction through monitoring the disappearance or appearance of a features IR absorption signal. For instance, the oxidation of a secondary alcohol to a ketone could be monitored within terms of the disappearance of the O-H signal in the IR spectrum of the reaction mixture or through the appearance of the carbonyl signal of the ketone being produced. A rate of such reactions could also be measured through taking the time dependant spectra and using the Beer-Lambert Law.
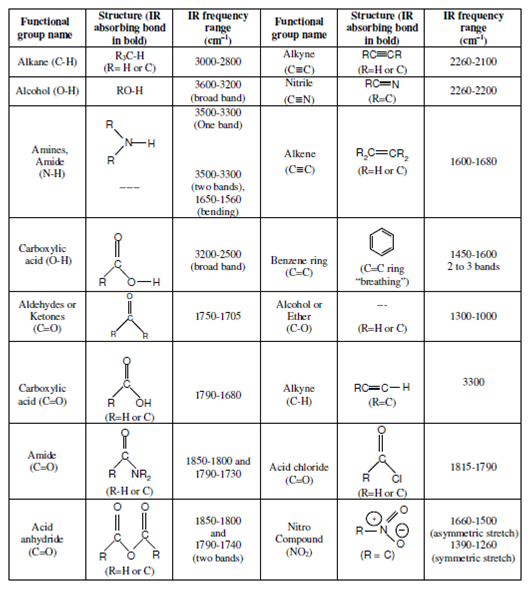
Table: Characteristic IR frequencies of some functional groups