Methodology of Quantitative Analysis:
As you would have understood through now in which the common procedure within flame photometric determination includes preparation of the sample solution in an appropriate solvent and aspirating it into the flame and measuring the intensity of the emitted radiation at the features wavelength of the element. From the radiation intensity measured, a concentration of the element within the sample is then determined. You have learnt that the energy emitted through the excited atoms within the flame could be associated to the number of atoms contained in the sample.
Therefore, flame photometry is not an absolute method; it is empirical and is sensitive to experimental conditions. For instance, the signal intensity from a flame is dependent on the flame temperature, a rate of flow of liquid within the flame, the pressure and rate of flow of fuel gases, and several other variables that affect the character of the flame or atomisation of the sample. Thus, we could acquire reliable results only through performing repeated checks of reproducibility and the effects of altering conditions. It is thus, necessary that the relation among the intensity of the emitted radiation and the concentration of the related element must be established. Instead we required to prepare a calibration curve as displays in Figure.
The calibration curve is prepared through measuring the intensity of emission for a series of solutions of various concentrations prepared through using a standard solution and plotting a graph among emission intensity versus concentration of the ionic species of the components of interest. The concentration of the element within the unknown sample could then be found out from the standard plot as is completed in visible spectrophotometry. In addition, it is extremely important to measure the emission from the standard and unknown solutions under conditions which are as nearly identical as probable.
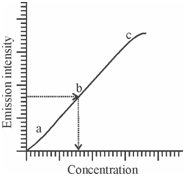
Figure: Calibration plot: Emission intensity versus Concentration of the analyte