Characteristic emission wavelengths:
Very frequent internal standard lithium is added to take care of matrix effects. For instance, within calcium analysis, La (III) or EDTA must be added to the solution to prevent interference because of phosphate. Food and drinks are commonly analysed through ashing and leaching out the ash along with mineral acid to leach out these elements. Fruit juices, Soft drinks, and alcoholic beverages could sometimes be analysed through using flame photometry.
Analysis of water from several sources is carried out to determine its suitability for washing, drinking, agricultural and industrial purposes. Analysis of effluent water from various organizations is carried out to suggest the probable methods of treatment of this waste water.
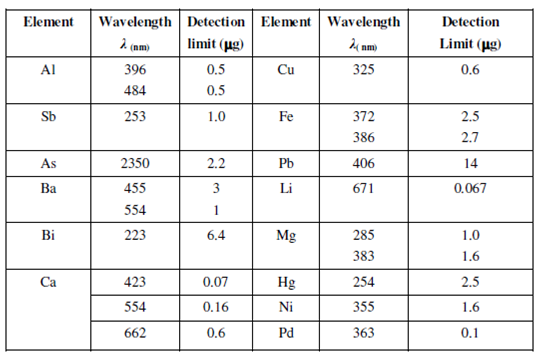
The applications enumerated above displays the importance of quantitative determinations of simple ions in huge range of samples. Such determinations are one of the most useful applications of flame photometry. Table lists some of the elements that can be determined through flame photometry and their detection limits.
Table: Elements, their characteristic emission wavelengths and detection limits
Let us learn about the methodology for quantitative determination by using flame photometry.