Application of Coulometry:
A coulometric technique has considerably greater scope than electrogravimetry. It is not as you have seen electrogravimetric methods are limited to the analysis of metals which can be plated out and weighed. Alternatively in coulometry methods do not require weighing (require current measurement) and reaction need not give rise to formation of a solid product. Additional, coulometric methods are also not limited to reduction of metal ions but could be used for any electrode procedure that could be operated at near 100 percent current effectiveness. For instance, controlled potential coulometry has been used to determine organic compounds, like as trichloroacetic acid and picric acid. Coulometric technique is not limited to reduction process only but reactions taken place at anode could also be studied.
In coulometry, at controlled potential, commonly a mercury cathode is preferred because the optimum controlled potential for a provided separation is simply determined from the polarographic data along with the DME. In common there is no benefits in employing a controlled potential more than - 0.15 V will higher than half-wave potential E?. A few values for the half-wave potential (E?) are listed in Table
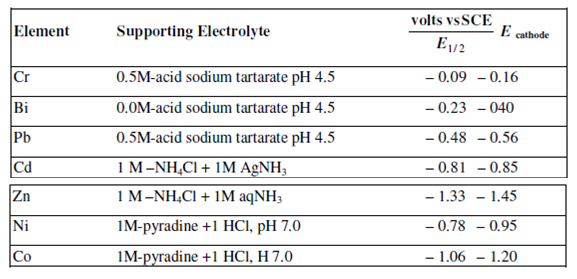
Through means of controlled cathode potential at methods, it is possible to determine simultaneously Cu and Bi, Cd and Zn and Ni and Co in a solution.