Effective dose, therapeutic index, and drug toxicity
Because of the intimate replication of viruses within cells, and their absolute reliance on cellular protein and energy metabolisms, the development of antiviral compounds faces the difficult challenge of selective toxicity – interference with viral replication needs to
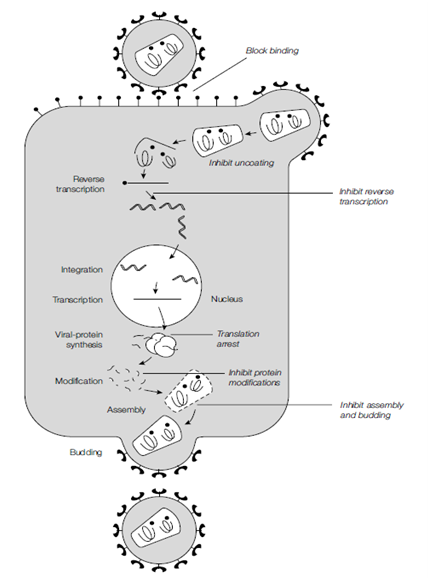
Figure: The various stages of virus replication. Antiviral drugs can be used to inhibit any of these steps.
be achieved without unacceptable damage to the host (uninfected cells). The activity of antiviral compounds is assessed at an early stage in their development, quantifying their ability to interfere with viral growth in tissue culture. The replication of the target virus is assayed by, for example, TCID50 or plaque assay, comparing levels of infection within cells in the presence of various concentrations of the drug candidate (with drug-free
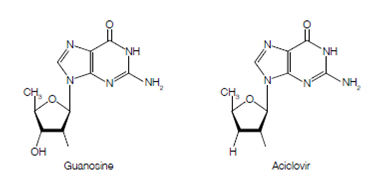
Figure Chemical structures of guanosine and aciclovir, revealing the exchange of a single hydroxyl (OH) group for hydrogen (H) within the ribose sugar ring.
control infections alongside). The resulting level of virus infectivity is plotted against the drug concentration and the concentration of compound that reduces virus titer by 50% is expressed as the effective dose 50 concentration (ED50); if achievable, the ED90 concentration is also determined. Tissue culture can also be used to assess the toxicity.
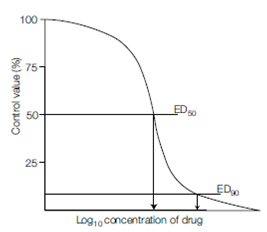
Figure: Determination of effective dose (ED50, ED90) for an antiviral drug in tissue culture.
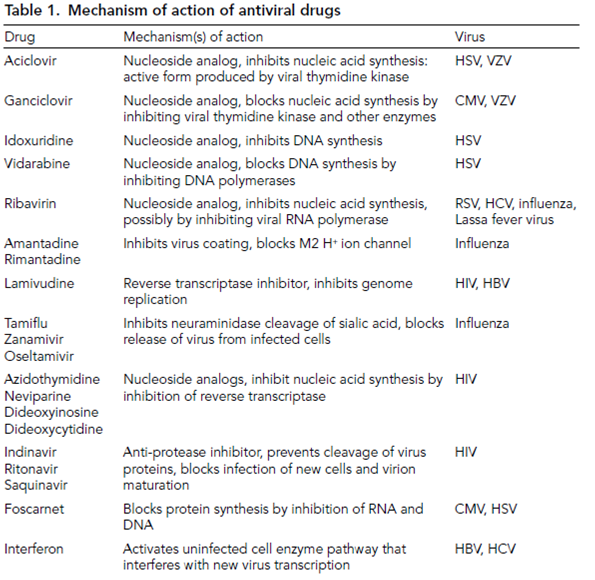
of compounds, although this is often tested in animals and humans. Animals play a vital role in the study of toxicity and a number of statutory tests are carried out to determine the risks and side effects before new compounds enter clinical trials. Drug toxicity is expressed as the selective index, which determines the ratio between the concentration at which the drug inhibits cell proliferation or DNA synthesis (i.e. it is toxic to uninfected cells), and the concentration at which the drug inhibits virus replication. A high value indicates a selective drug with low toxicity, while a ratio approaching equivalence indicates that the compound is toxic. For compounds with a less than ideal selective index, a consideration of benefit/risk ratio can be appropriate, as side effects may be tolerated more if the risk from the disease is high (e.g. AIDS). Some antiviral agents have a level of toxicity which, while acceptable for some diseases (e.g. AIDS), would not be tolerated for less severe diseases.