ELISA
Specific antibodies can also be used to measure the amount of the corresponding antigen in a biological sample. Various kinds of immunological assays exist. A popular edition is enzyme-linked immunosorbent assay is ELISA that can readily detect and quantify less than a nanogram of a specific antigenic protein. In the ELISA specific antibody is coupled to a solid support. The convenient format for ELISA is to use a plastic tray that has molded wells in it a microtiter tray where the antibody has been coupled to the plastic forming the wells. For Samples to be assayed are added to the wells. If antigen is present which is recognized through the antibody, it becomes bound in figure. The wells are then washed to remove unbound protein and incubated with a second antibody that recognizes the protein but at a unique epitope than the first antibody. The second antibody is attached to an enzyme which can catalyze the conversion of a colorless or nonfiuorescent substrate into a fiuorescent or colored product. The intensity of the color or fiuorescence produced for every sample is then measured to determine the amount of antigen present in every sample. Various machines are commercially available which scan the wells of microtiter plates following ELISA and quantify the amount of antigen bound in every well.
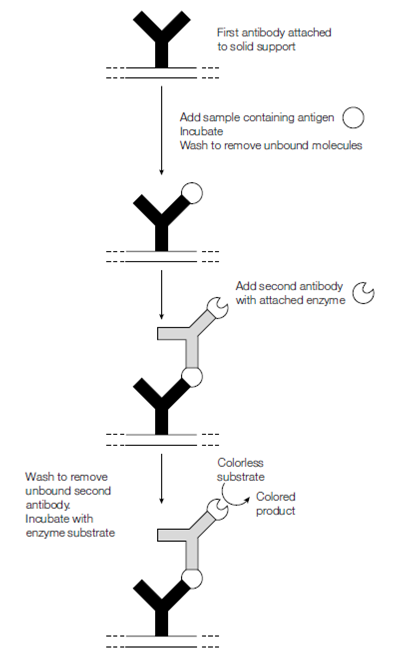
Figure: ELISA using a second antibody with an attached (conjugated) enzyme that converts a colorless substrate to a colored product.
Immunoblotting This method can be used for detection of one or more antigens in a combination.The sample is electrophoresed on an SDS–polyacrylamide gel which separates the proteins on the basis of size resulting in a sequence of protein bands down the gel. Because the gel matrix does not let huge
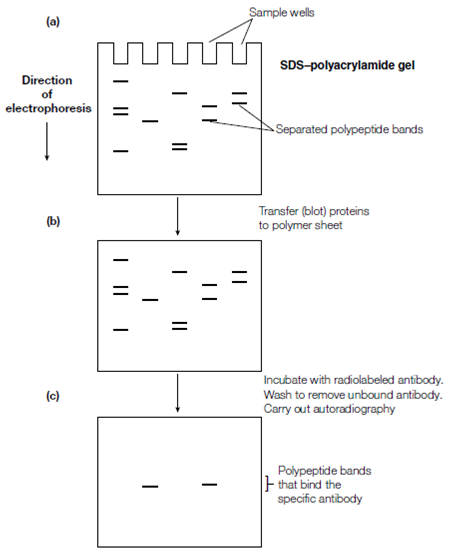
Figure: Immunoblotting using a radiolabeled antibody (a) SDS polyacrylamide gel electrophoresis, yielding polypeptides separated into discrete bands; (b) nitrocellulose or nylon membrane onto which the protein bands have been blotted (i.e. a Western blot); (c) autoradiograph after incubating the Western blot with radiolabeled antibody, washing away unbound antibody and placing the membrane against X-ray film.
proteins like as antibodies enter readily the sample proteins must be first transferred to a more accessible medium. This procedure is called blotting. The gel is placed next to a nitrocellulose or nylon sheet and an electric field is applied so in which proteins migrate from the gel to the sheet where they become bound. This particular form of blotting for example blotting of proteins is called Western blotting to differentiate it from blotting of DNA or. To detect specific proteins (antigens) by using antibody probes (immunoblotting), the Western blot is incubated with a protein such as casein to bind to nonspecific protein-binding sites and hence prevent spurious binding of antibody molecules in subsequent steps.
This step is said to ‘block’ nonspecific binding sites. The Western blot is then reacted with labeled antibody, unreacted antibody is washed away and those protein bands that have bound the antibody become visible and hence are identified. The method of visualization depends on how the antibody was labeled. If the labeling is by the incorporation of a radiolabel for example 125I, then autoradiography is carried out to detect the radioactive protein bands. Alternatively, the antibody may be detected by incubating the sheet with a second antibody that recognizes the first antibody for instance if the first specific antibody was raised in rabbits, the second antibody could be a goat anti-rabbit anti- body. The second antibody could be radiolabeled and its binding detected by autoradiography or it could be conjugated to an enzyme that generates a colored product as in ELISA. Immunoblotting can also be used to analyze specific antigens after two-dimensional gel electrophoresis which resolves proteins as spots, separated on the basis of both charge and size.