Variable and constant regions
Contrast of the amino acid series of several immunoglobulin polypeptides has shown in which each light chain has a variable region at its N-terminal end and an invariant or constant region at its C terminus. Similarly every heavy chain has an N-terminal variable region and a C-terminal constant region. Because it is the N-terminal component of the light and heavy chains which form the antigen-binding site the variability in amino acid series of these regions explains how hard sites with various specificities for antigen binding can be established. In real, the variability in the variable regions of both heavy and light chains is commonly localized to three hypervariable regions in each chain. In the 3D structure of the immunoglobulin molecule, the hyper- variable component of the light and heavy chains are looped together to build the antigen-binding site. The remaining parts of every variable region stay reasonably constant in sequence, generally do not contact the antigen directly and are called framework regions.
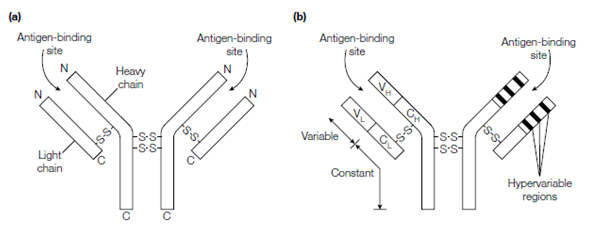
Figure: Structure of an antibody molecule. (a) Each antibody molecule consists of two identical light chains and two identical heavy chains.
The molecule has two antigen-binding sites, each formed by a light chain and a heavy chain. (b) The N-terminal regions of the light and heavy chains are variable in amino acid sequence from antibody to antibody (variable regions; V regions) whilst the C-terminal regions are relatively constant in sequence (constant regions; C regions). The generic terms for these regions in the light chain are VL and CL and for the heavy chains are VH and CH.